The CREXONT Difference
CREXONT features a novel ER technology1

How CREXONT works
CREXONT® (carbidopa and levodopa) extended-release capsules contain a mucoadhesive polymer that no other oral CD/LD treatment provides1‑3
| IR CD/LD | RYTARY® | CREXONT® | |
|---|---|---|---|
| Immediate-release (IR) component | |||
| Extended-release (ER) component | |||
| Mucoadhesive polymer technology |
Discover the novel mechanism of action of CREXONT
For the first time in Parkinson’s disease, an oral therapy was designed to target where levodopa is best absorbed—the proximal small intestine—making CREXONT the oral CD/LD therapy with the longest-lasting LD plasma levels.1,4,5*†
*Exact site and duration of absorption are unknown.
†Based on the time that LD plasma levels were maintained above 50% of Cmax.6
CREXONT combines immediate-release (IR) granules and extended-release (ER) pellets1,7





LD plasma levels
Capsule and contents shown for illustrative purposes only.
The ER technology is designed to1‡:
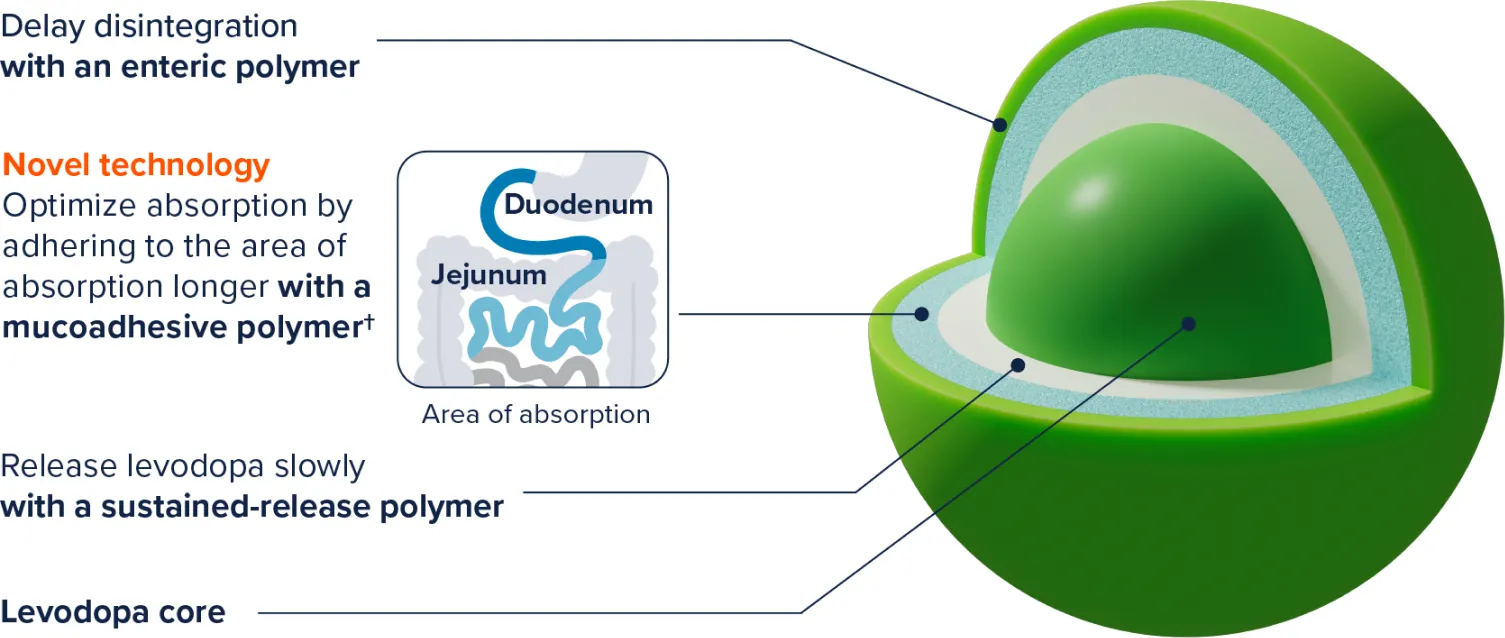
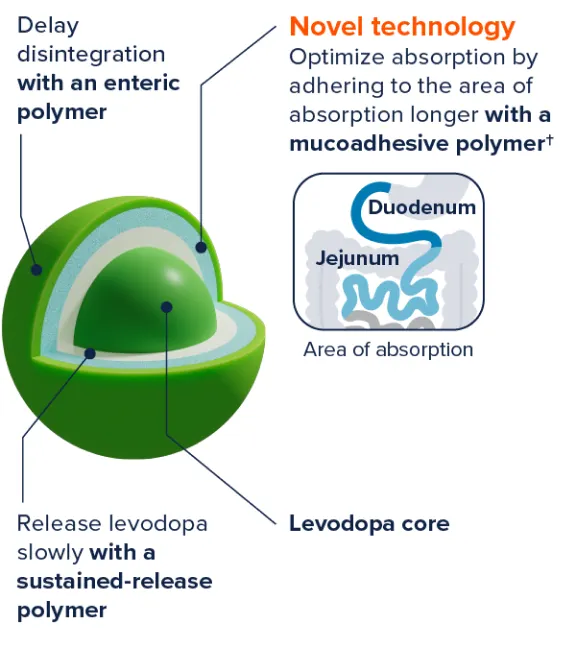
‡Exact mechanism is unknown.
§Exact site and duration of absorption are unknown.
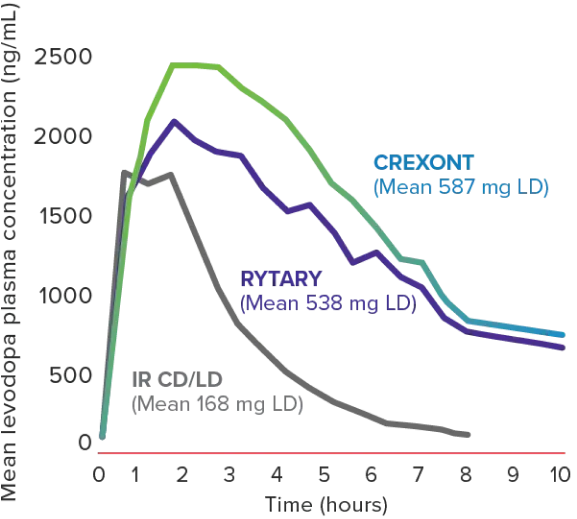
Pharmacokinetic study6
CREXONT LD plasma levels lasted longer than other oral CD/LD formulations in patients with PD1,6*
Primary endpoint: pharmacokinetic profile8
Mean LD plasma concentration-time profiles following a single dose of CREXONT, RYTARY, and IR CD/LD in patients with advanced PD (n=24)6,8†‡§
Dose selection
The dose of IR CD/LD was the patient’s prestudy morning baseline dose, while the doses of CREXONT and RYTARY were chosen based on previous PK findings in healthy subjects.6
Post hoc analysis of a prespecified secondary PK parameter: duration of LD concentration >50% of Cmax8
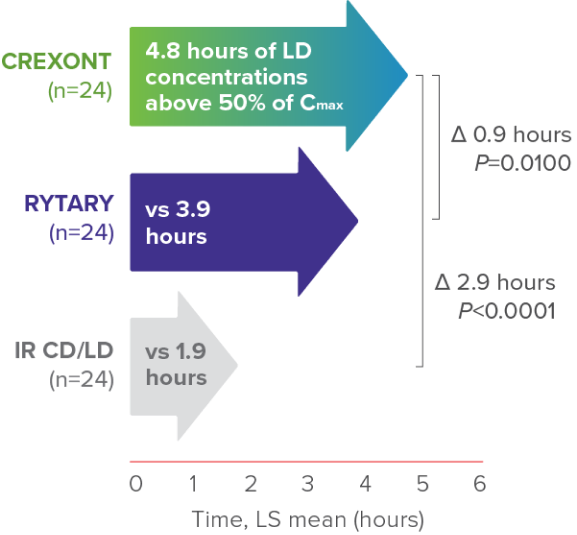
CREXONT LD plasma levels were sustained for longer than those of RYTARY and IR CD/LD6,8*
*Based on the time that LD plasma levels were maintained above 50% of Cmax.6
†Assessment performed on patients in a fasted and “Off” state.6
‡No plasma concentration values were available after the 8-hour time point in the IR CD/LD group because all patients in that group had been rescued by then.8
§Summary statistics for LD PK parameters are presented by treatment, across all doses of CREXONT, IR CD/LD, and RYTARY, respectively: Cmax (mean ± SD): 3161 ± 1665 ng/mL, 2492 ± 1459 ng/mL, 2839 ± 1909 ng/mL; tmax (median [min–max]): 2.0 h (0.5–7.0); 1.0 h (0.5–2.5); 2.0 h (0.5–6.5); t1/2 (mean ± SD): 2.3 ± 0.9 h, 1.4 ± 0.3 h, 2.0 ± 0.7 h; AUCt (mean ± SD): 13,291 ± 7264 ng∙h/mL, 4879 ± 2631 ng∙h/mL, 10,467 ± 6771 ng∙h/mL; AUC0–∞ (mean ± SD): 16,734 ± 9759 ng∙h/mL, 5456 ± 2896 ng∙h/mL, 13,840 ± 8899 ng∙h/mL.8
AUC0–∞=area under the curve extrapolated to Time infinity; AUCt=area under the curve until the last observation Time t; CD/LD=carbidopa/levodopa; Cmax=maximum observed plasma concentration; IR=immediate-release; LD=levodopa; LS=least squares; PD=Parkinson’s disease; PK=pharmacokinetic; SD=standard deviation; t½=half-life; tmax=time to maximum concentration.
Review the head-to-head study results evaluating CREXONT vs IR CD/LD
IMPORTANT SAFETY INFORMATION
Indications and Usage
CREXONT® (carbidopa and levodopa) extended-release capsules for oral use is indicated for the treatment of Parkinson’s disease, post-encephalitic parkinsonism, and parkinsonism that may follow carbon monoxide intoxication or manganese intoxication in adults.Dosage and Administration
- Levodopa-naïve patients: Starting dose is 35 mg carbidopa/140 mg levodopa taken orally twice daily for the first three days; thereafter, dosage may be increased gradually as needed
- For patients converting to CREXONT from immediate-release carbidopa/levodopa, dosages are not substitutable on a 1:1 basis. See full prescribing information Section 2.2 for instructions
- For patients converting from Rytary® (carbidopa and levodopa) extended-release capsules, initiate CREXONT on an approximately 1:1 mg basis using the levodopa component for conversion
- CREXONT may be taken up to four times daily. The maximum recommended daily dosage is 525 mg carbidopa/2100 mg levodopa
- CREXONT may be taken with or without food. Capsules should not be chewed, divided or crushed
- CREXONT should not be taken with alcohol
Contraindications
Nonselective MAO inhibitors.Warnings and Precautions
- CREXONT may cause falling asleep during activities of daily living, somnolence or dizziness. Patients should avoid activities that require alertness such as driving and operating machinery until they know how CREXONT affects them
- It is important to avoid sudden discontinuation or rapid dose reduction to reduce the risk of withdrawal symptoms such as high fever or confusion. Patients who are discontinuing CREXONT should taper off with healthcare provider guidance
- Consider dose reductions or stopping CREXONT in patients with hallucinations or impulse control disorders (e.g., gambling, sexual urges, or uncontrolled spending)
- Consider dose reduction in patients with dyskinesia
- Patients with a major psychotic disorder should not be treated with CREXONT
- Monitor patients with a history of cardiovascular disease for cardiac function
- Monitor patients with a history of peptic ulcer for upper GI hemorrhage
- Monitor patients with glaucoma for increased intraocular pressure
Adverse Reactions
The most common adverse reactions (incidence ≥ 3% and greater than immediate-release CD/LD) are nausea and anxiety.Drug Interactions
Iron salts and dopamine D2 antagonists, including metoclopramide, may reduce the effectiveness of CREXONT.Use in Specific Populations
Pregnancy: Based on animal data, CREXONT may cause fetal harm. There are no adequate data on the developmental risk associated with the use of CREXONT in pregnant women.Breastfeeding: The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for CREXONT.
Geriatric patients: There were no differences in safety outcomes between patients less than 65 years of age, 65-75 years of age, or 75 years and older.
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Specialty, a division of Amneal Pharmaceuticals, LLC at 1‑877‑835‑5472 or the FDA at 1‑800‑FDA‑1088 or www.fda.gov/medwatch.
Please see full Prescribing Information for CREXONT.
Content is for guidance only. Please use clinical judgment when prescribing CREXONT. Dosage is individualized for each patient.
References: 1. Hauser RA, Espay AJ, Ellenbogen AL, et al. IPX203 vs immediate-release carbidopa-levodopa for the treatment of motor fluctuations in Parkinson disease: the RISE-PD randomized clinical trial. JAMA Neurol. 2023;80(10):1062-1069. 2. SINEMET [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2020. 3. Mittur A, Gupta S, Modi NB. Pharmacokinetics of Rytary®, an extended-release capsule formulation of carbidopa–levodopa. Clin Pharmacokinet. 2017;56(9):999-1014. 4. LeWitt PA. Levodopa therapy for Parkinson’s disease: pharmacokinetics and pharmacodynamics. Mov Disord. 2015;30(1):64-72. 5. Tambasco N, Romoli M, Calabresi P. Levodopa in Parkinson’s disease: current status and future developments. Curr Neuropharmacol. 2018;16(8):1239-1252. 6. Modi NB, Mittur A, Rubens R, Khanna S, Gupta S. Single-dose pharmacokinetics and pharmacodynamics of IPX203 in patients with advanced Parkinson disease: a comparison with immediate-release carbidopa-levodopa and with extended-release carbidopa-levodopa capsules. Clin Neuropharmacol. 2019;42(1):4-8. 7. CREXONT [package insert]. Bridgewater, NJ: Amneal Pharmaceuticals LLC; 2024. 8. Data on file. Amneal Pharmaceuticals LLC.

