Laura’s journey with Parkinson’s began long before she could put a name on it. Some of its many and diverse symptoms popped up occasionally years before her diagnosis—freezing mid-stride while crossing a busy avenue; sobbing for no reason during a meeting with a client; walking with her right forearm stuck halfway up, as if ready to shake hands. But these manifestations were so diverse that it took many doctors and two surgeries to figure out. When she finally received her Parkinson’s diagnosis in 2014, it was a moment of both shock and clarity.
Living with Parkinson’s meant adapting, but Laura wasn’t someone who saw adaptation as defeat, but as a way forward. With a background in literature, advertising, and public relations, she began using design as a tool to navigate what her new normal looked like. She sketched out visual guides, timelines of her symptoms, color-coded medication trackers, and drawings that captured the emotional toll of Parkinson’s on her life. These weren’t just for her, they became resources for others in her support group.

Still, one of the biggest challenges she faced was medication management. For years, Laura was taking immediate-release medications on a strict schedule. It dominated her day. Every meal and activity had to be planned.
Over time, she began to feel like she was managing her medication more than she was managing her disease.

That changed when she started CREXONT® (carbidopa and levodopa) extended-release capsules. Laura was immediately interested in the idea of a long-acting formulation. She knew the science. She’d tracked her own response patterns in detail. And she was ready for something that might offer more stability.
With CREXONT, Laura felt like her days started to flow more naturally again. Instead of constantly anticipating her next dose, she began moving through her routine with more confidence. The extended-release formulation gave her stretches of “Good On” time, which is when your medicine is working well, and you aren’t experiencing symptoms. It also means you’re not having those movements you can’t control, called dyskinesia, which include twitches, jerks, and twisting motions. Or, if you are experiencing them, they’re not affecting your daily activities.
She’s returned to things she loves, including dancing, painting, and traveling. Recently, she took a cruise with her husband and is planning another with her great grandchildren. She will be packing her CREXONT pill containers, which she has marked with color-coded stickers to match her personalized schedule.
As a support group leader, Laura often shares her experience with CREXONT as an example of how finding the right treatment for you can bring back ease, flow, and confidence.
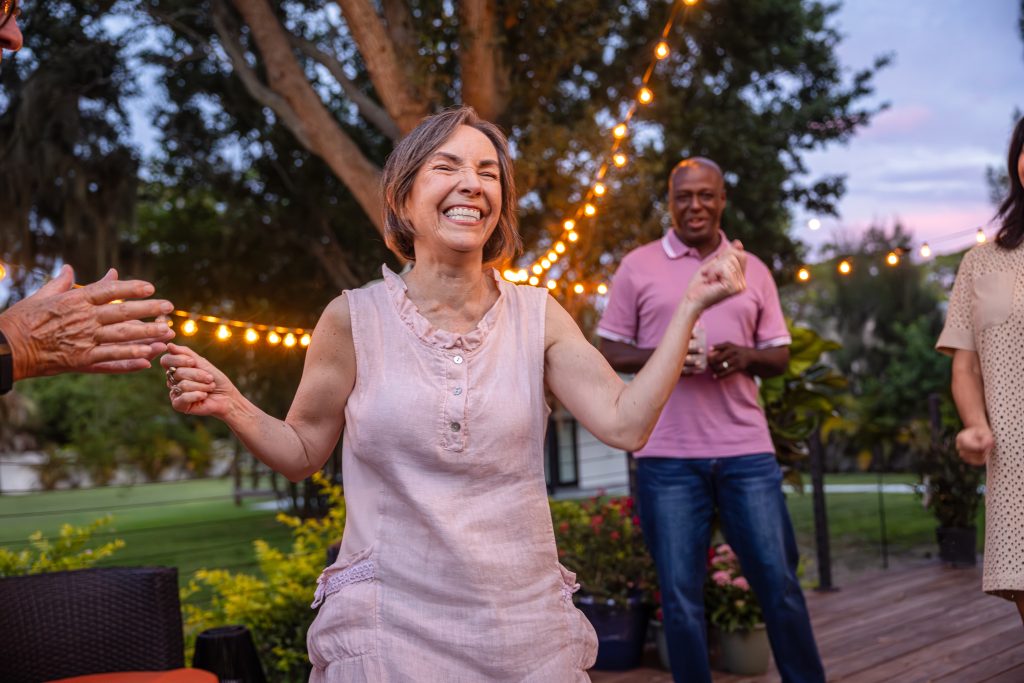
When she’s not hosting group discussions or traveling, Laura is building a toolkit she hopes to publish one day. It is a visual diary and planning system for others living with Parkinson’s, complete with illustrations, symptom journals, and daily affirmations.
Laura’s experience with CREXONT is her own and may not reflect the experience of every patient. For some patients, CREXONT may cause falling asleep during daily activities. Side effects may include nausea and anxiety. Individual results will vary. Talk to your patients to see if CREXONT is right for them. Only an HCP should assess each patient’s condition and advise them on treatment options.




